3 Pharmaceutical Stocks For Your Watchlist Right Now
For investors wondering what are the best stocks to invest in today, pharmaceutical stocks would be an interesting play. On the whole, this part of the stock market is home to companies of varying sizes and specializations. The likes of which serve to aid people across the globe experiencing various illnesses and diseases. Pair this with the current uncertainty in markets and it would not be too surprising to see investors considering pharmaceutical stocks today.
Take Ikena Oncology (NASDAQ: IKNA) for instance. All in all, the company just announced today that the Food and Drug Administration (FDA) has granted a Fast Track designation for IK-930. This designation is to accelerate the development and review of drugs targeted for serious conditions with unmet medical needs. In particular, the IK-930 is a novel TEAD inhibitor which targets the HIPPO signaling pathway, in patients with unresectable NF2-deficient malignant pleural mesothelioma (MPM). In essence, MPM is a rare type of cancer that resides in the lungs and chest cavity.
At the same time, relatively larger firms like BioMarin Pharmaceutical (NASDAQ: BMRN) are also making headlines. As of yesterday, the Ministry of Health, Labor and Welfare of Japan is granting approval for the registration of BioMarin’s Voxzogo treatment. In brief, Voxzogo is a modified C-type natriuretic peptide that directly targets the underlying pathophysiology of achondroplasia. Furthermore, Voxzogo will be used for injection to treat achondroplasia in children of all ages. If all this has you keen on pharmaceutical stocks, here are three more to watch in the stock market today.
Pharmaceutical Stocks To Watch Today
- AbbVie Inc. (NYSE: ABBV)
- ANI Pharmaceuticals Inc. (NASDAQ: ANIP)
- Merck & Co. Inc. (NYSE: MRK)
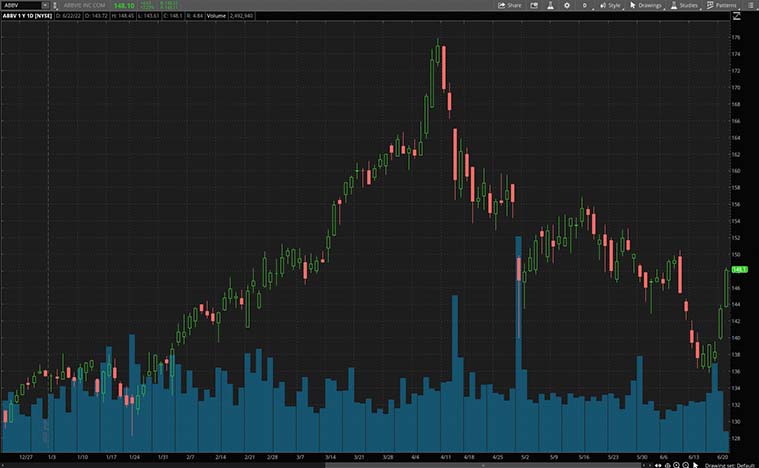
AbbVie Inc.
AbbVie is a biopharmaceutical company that was a spin-off from Abbott Laboratories (NYSE: ABT). Accordingly, the company discovers, develops, and commercializes advanced therapies that continue to improve the quality of life and save millions of lives. Today, it has about 50,000 employees working all around the globe to help patients. On Tuesday, the company announced an exciting piece of news.
Diving in, it announced that it has submitted a supplemental New Drug Application (sNDA) for atogepant to the FDA. Atogepant supports the preventive treatment of chronic migraine in adults. If approved, atogepant would be the first gepant (oral calcitonin gene-related peptide receptor antagonist) for the broad indication of the preventive treatment of migraine, including episodic and chronic. “Having one oral medication to treat both episodic and chronic migraine would be an important advancement for health care providers and patients,” said Michael Gold, M.D., therapeutic area head, neuroscience development, AbbVie. “This sNDA approval would also diversify AbbVie’s migraine portfolio and make it the only company to offer two approved preventive treatments for those living with chronic migraine. No two migraine patients are alike, so having multiple treatment options with unique mechanisms of action is critical.”
The company seems to be firing on all cylinders, as it had received FDA approval for Risankizumab-rzaa last week. It is the first and only specific interleukin-23 (IL-23) inhibitor for the treatment of adults with moderately to severely active Crohn’s disease. In two induction and one maintenance clinical trial, Risankizumab-rzaa demonstrates significant improvements in endoscopic response. All things considered, is ABBV stock worth watching right now?
[Read More] Stock Market Today: Dow Jones, S&P 500 Slide After Tuesday’s Rally; La-Z-Boy Up On Upbeat Quarter
ANI Pharmaceuticals Inc.
Next, we have ANI Pharmaceuticals, a diversified biotech company that serves patients by developing and manufacturing high-quality branded and generic prescription pharmaceutical products. It also focuses on delivering sustainable growth by building on its successful Purified Controphin Gel franchise. The company has a fully automated solid dose packaging line that offers a complete assembly from bottle unscrambling to bundling.
Getting into today’s news, ANI stock could be in focus following the company’s latest FDA nod. Namely, ANI’s Clorazepate Dipotassium tablets now have FDA approval for the Abbreviated New Drug Application (ANDA). With an ANDA, the company can now manufacture and market the treatment. According to CEO Nikhil Lalwani, this serves to strengthen ANI’s base business. Overall, Lalwani also says, “The imminent launch and commercialization of Clorazepate Dipotassium Tablets is another example of our ongoing commitment to ensuring that patients in need have access to important therapeutics.”
On June 21, 2022, the company announced a collaboration with Veeva Systems (NYSE: VEEV) to define and operationalize data-driven commercial strategies for ANI’s new rare disease business unit. Through this collaboration, ANI will have a stronger digital foundation that supports its patient-centric approach to commercialization. With that being said, is ANI stock a top pharmaceutical stock to consider investing in?

[Read More] Recession-Proof Stocks To Invest In Now? 3 E-Commerce Stocks To Watch
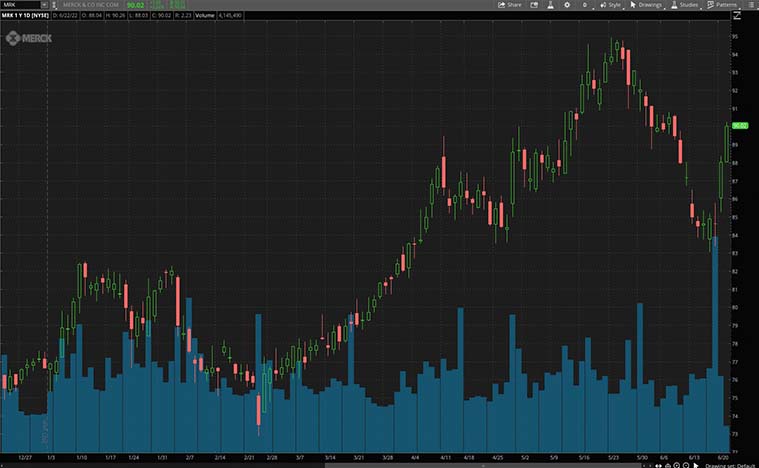
Merck & Co. Inc.
Last but not least, we have the global biopharmaceutical company, Merck. In short, the company offers health solutions through its prescription medicines, vaccines, biological therapies, and animal health products. In particular, the company’s two key operating segments are Pharmaceutical and Animal Health. All in all, the Merck’s Pharmaceutical segment includes human health pharmaceuticals and vaccine products. Meanwhile, the Animal Health segment creates, manufactures, and sells a variety of veterinary pharmaceutical and vaccination products.
Today, Merck has announced that the FDA has approved an expanded indication for Vaxneuvance. Also known as the Pneumococcal 15-valent Conjugate Vaccine, it is used for the prevention of pneumococcal disease in infants and children aged 6 weeks to 17 years. This FDA approval was based on data from seven randomized, double-blind clinical studies. In essence, a four-dose pediatric series of Vaxneuvance was found to be non-inferior to the 13-valent pneumococcal conjugate vaccine.
Yesterday, Merck and AstraZeneca (NASDAQ: AZN) announced that the results of the Phase 3 PROpel trial have been published in NEJM Evidence. In conclusion, the trial results showed that Lynparza in combination with abiraterone plus prednisone significantly improved radiographic progression-free survival (rPFS). This is compared to the abiraterone plus prednisone, the standard of care, as a first-line treatment for patients with metastatic castration-resistant prostate cancer (mCRPC) with or without HRR gene mutations. Considering the positive outlook, would MRK stock be worth adding to your watchlist?
If you enjoyed this article and you’re interested in learning how to trade so you can have the best chance to profit consistently then you need to checkout this YouTube channel. CLICK HERE RIGHT NOW!!